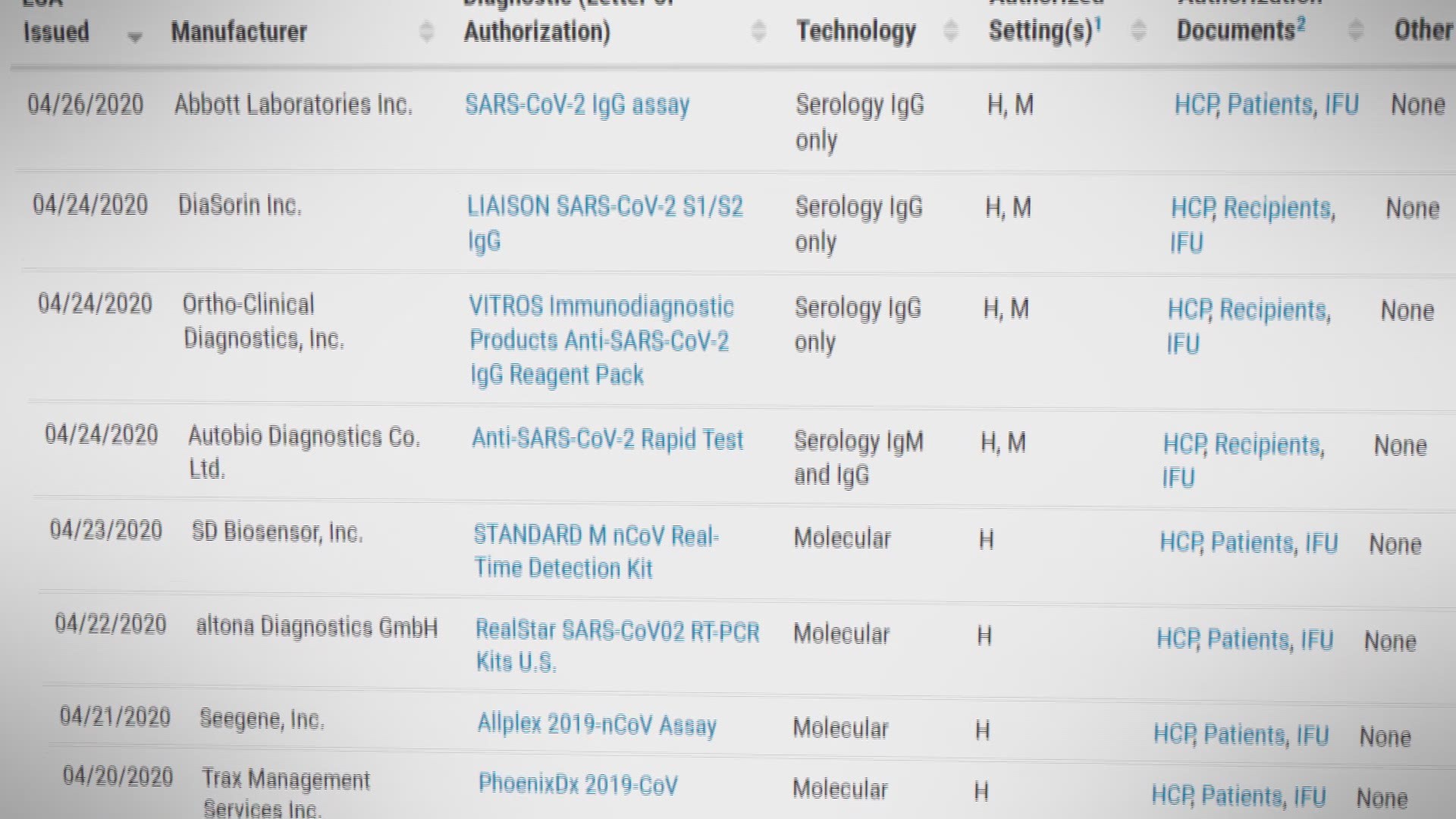
WASHINGTON — The Food and Drug Administration has issued an emergency authorization for a COVID-19 antigen test. The company claims it can provide results in 15 minutes. But, such tests reportedly have reliability drawbacks.
Quidel Corporation announced the emergency use authorization Friday for the Sofia 2 SARS Antigen FIA.
“The EUA for our Sofia 2 SARS Antigen FIA allows us to arm our healthcare workers and first responders with a frontline solution for COVID-19 diagnosis, accelerating the time to diagnosis and potential treatment of COVID-19 for the patient," Quidel CEO Douglas Bryant said in a statement.
An antigen is a substance that causes the body to make an immune response against that substance, according to the National Cancer Institute. A virus is one such substance.
CNBC reports antigen tests can provide results in minutes, but they cannot detect all active infections. While antigen tests that show a positive result are highly accurate, they can also provide a false negative.
On the flip side, polymerase chain reaction (PCR) tests are more accurate but take longer to achieve results. A negative antigen test result would need to be confirmed with a PCR test.
The antigen test could be used on millions of Americans daily once production ramps up, according to CNBC. They cost less than PCR tests and have a simpler design.
Lack of widespread testing has been a hindrance in fighting the spread of the coronavirus.
There have been 8.4 million tests performed for COVID-19 since the first confirmed case was announced nearly four months ago, according to Johns Hopkins University. There have been nearly 1.3 million cases as of Saturday morning. More than 77,000 Americans have died while nearly 200,000 have recovered.