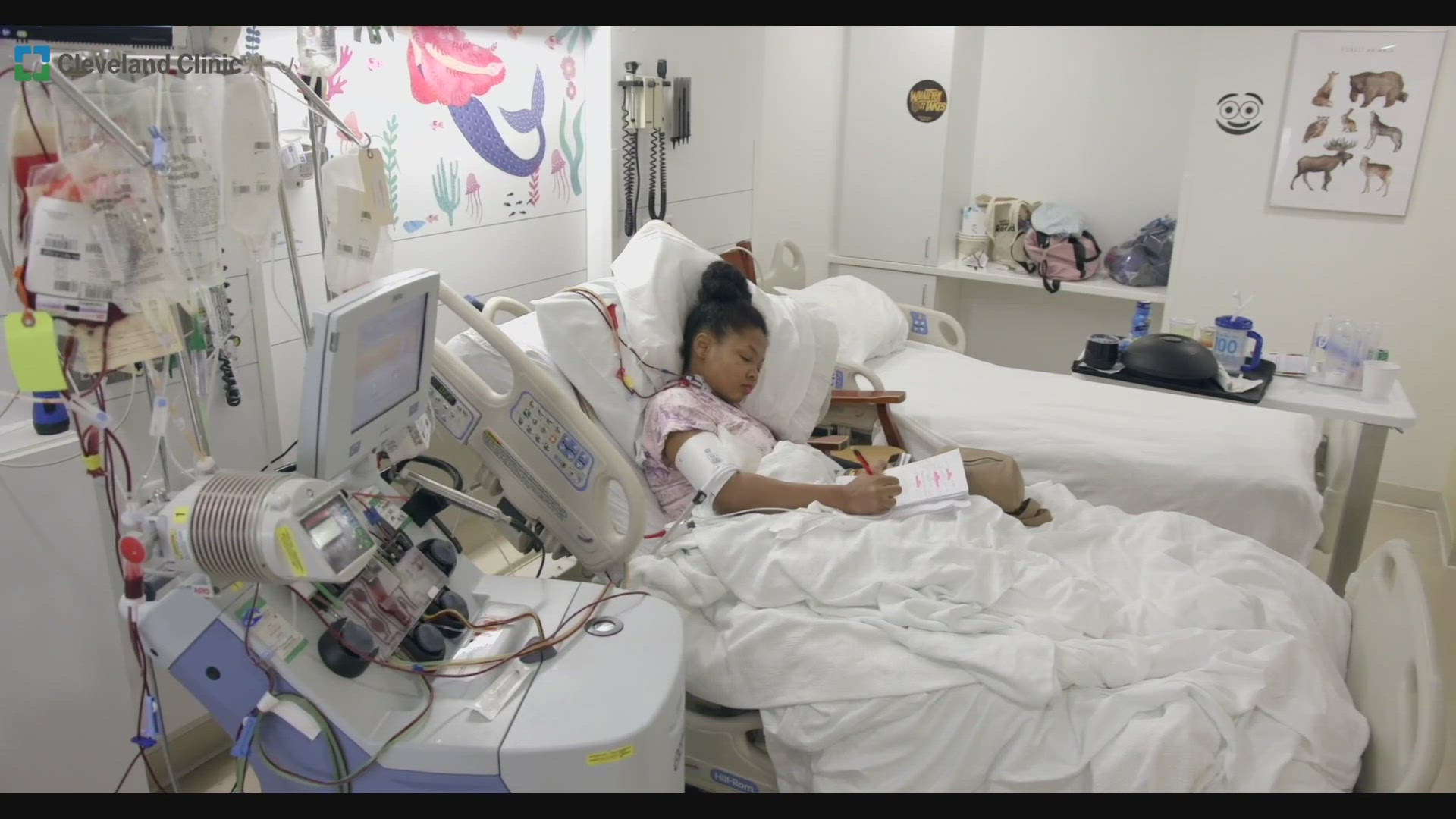
CLEVELAND — 34-year-old Danielle Lee says she remembers most of her young life in and out of Cleveland hospitals every time she had a painful sickle cell episode. As the years went on those hospital stays became longer and the pain became progressively worse, despite the standard protocols of treatment.
The disease took her spleen, gall bladder and damaged her hip joints and bones. So when she was offered an opportunity to try a new gene editing therapy that is a one time treatment that re-engineers her stem cells to correct the mutation that caused her sickle cell disease, she signed up.
She knew the treatment couldn't fix the damage already done, but she never thought she'd hear the words functional cure.
"It's such a blessing, God is good, that's all I can say," Danielle said.
Last week, Researchers presented the latest findings from the clinical trial Danielle is part of called the RUBY Trial.
Conducted as part of the multicenter RUBY Trial, researchers shared data on the safety and effectiveness of renizgamglogene autogedtemcel (reni-cel, formerly known as EDIT-301), an experimental one-time gene editing cell therapy, among its 18 patients at the European Hematology Association 2024 Hybrid Congress (EHA) in Madrid, Spain.
This innovative treatment modifies a patient’s own blood-forming stem cells to correct the mutation responsible for sickle cell disease.
The 18 patients – two of whom, including Danielle, were treated at Cleveland Clinic Children’s – underwent a procedure where their stem cells were first collected for gene editing. They then received chemotherapy to clear remaining bone marrow, making room for the repaired cells which were later infused back into their body.
The treatment was well-tolerated with no serious side effects reported. Following treatment, all patients successfully regained their white blood cells and platelets. Importantly, all patients have remained free of painful events since treatment, and those followed for five months or greater have seen their anemia resolve.
“It’s encouraging that this gene-editing treatment continues to show promising efficacy for sickle cell patients,” said Rabi Hanna, M.D., chairman of the division of pediatric hematology oncology and blood and marrow transplantation at Cleveland Clinic Children’s and the RUBY trial’s presenting investigator. “These latest results offer hope that this new experimental treatment will continue to show progress and get us closer to a functional cure for this devastating disease.”
Since her treatment, Danielle moved to LA where she's taking classes and hopes to become an actor and film maker. "I want to have the ability to act and do my own stunts like Tom Cruise, " she said smiling. Being free of sickle cell disease may give her a fighting chance.
In the United States, between 1 million to 3 million people carry the sickle cell trait, while approximately 100,000 individuals have sickle cell disease. This trait and disease are more common among certain ethnic groups, including African Americans, where approximately 1 in every 365 babies are born with sickle cell disease. It's also found in Hispanic populations.
Sickle cell disease is a genetic blood disorder that causes red blood cells to be misshapen like a sickle. Normal red blood cells are round and carry oxygen smoothly through blood vessels. In sickle cell disease, the abnormal cells block blood flow and break apart easily, leading to problems like severe pain, liver and heart issues, and a shorter life span, typically in the mid-40s. Medications can help manage the disease, but a cure is possible only through a blood or marrow transplant, which has risks and often requires a sibling donor.
The RUBY Trial is sponsored by Editas Medicine.