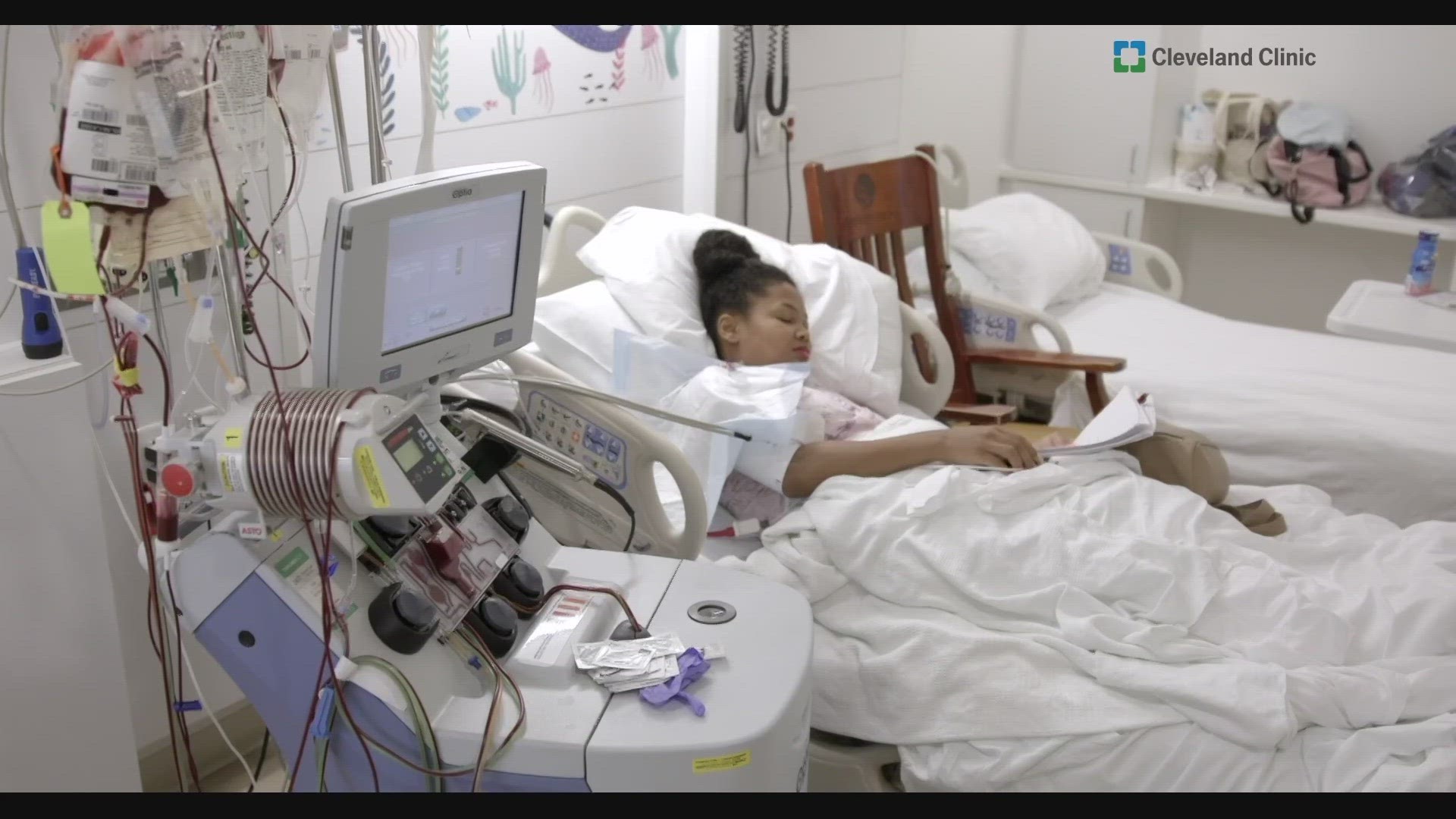
GARFIELD HEIGHTS, Ohio — Danielle Lee of Garfield Heights is an aspiring actress and a self-proclaimed adrenaline junkie. Although that doesn't mix with the sickle cell disease she's been fighting since birth.
The 33-year-old's dream is to write, star and direct movies where she can do her own stunts.
But several times a year she had a sickle cell crisis situations that often put her in the hospital in debilitating pain.
"Every crisis is potentially life threatening, when I was in school I had three to four crisis a year that landed me in the hospital and several others that didn't," Danielle said.
Sickle cell disease is an inherited disorder that affects the red blood cells. It’s more common in certain populations, including people who are African American, Hispanic, South Asian, Southern European Caucasian and Middle Eastern.
Traditionally, red blood cells are round and can move through small blood vessels to deliver oxygen. However, in people with sickle cell disease, the genetic change in DNA causes a chemical alteration in hemoglobin and alters the shape of red blood cells into a sickle like shape.
Danielle's disease caused more than pain, she also experienced avascular necrosis, which happens when blood flow to your bone tissue is blocked. She underwent multiple surgeries, including a core decompression where small holes are drilled in the affected bone to improve blood flow. She was driven to do more and didn’t want to have any limitations because of sickle cell disease.
That’s one of the reasons Dr. Rabi Hanna thought she could benefit from a clinical trial to test a new gene therapy treatment.
But Danielle wasn't convinced. Until she watched "The Gray Man" on Netflix. The film is about a CIA agent who uncovers agency secrets and is then hunted around the world. Danielle was mesmerized by the stunts, until she realized each one would have triggered an episode and likely put her in the hospital.
That's when she realized participating in the trial might bring her closer to her Hollywood dream.
It's been eight months since she's had her edited stem cells infused and she says so far she hasn’t had to be hospitalized for acute pain crisis.
Most recently she caught COVID-19. She was terrified of the virus during the pandemic because it could be devastating to those with SCD, but so far she's just experiencing a typical cold.
Although she still experiences some pain from the damage already done by sickle cell disease, she can relieve it with over-the-counter medication.
With the potential for gene editing to be a viable therapy, Danielle hopes other people with the condition will have the opportunity and access to the treatment.
"There are risks associated with it and it is a trial, so above all make sure you're doing it for yourself," Danielle said.
Researchers involved in the multicenter RUBY Trial presented an update on the safety and effectiveness of a single dose of EDIT-301, an experimental one-time gene editing cell therapy that modifies a patient’s own blood-forming stem cells to correct the mutation responsible for sickle cell disease.
Results are being presented at the European Hematology Association Hybrid Congress in Frankfurt, Germany.
The first four patients, two of whom are participating in the RUBY trial at Cleveland Clinic Children’s, had their stem cells collected for gene editing. The patients then underwent chemotherapy treatment to destroy their remaining bone marrow, making room for the repaired cells that were later infused back into their body.
This is the first time a novel type of CRISPR gene-editing technology – known as CRISPR/CA12 - is being used to edit human cells in a clinical trial. This technology is a highly precise tool to modify blood stem cells genomes that can potentially enable robust, healthy blood cell production.
The data showed new white blood cells in all four patients at about four weeks with no severe adverse effects. Patients also achieved a normal level of hemoglobin, which is the most important component of red blood cells that carry oxygen throughout the body.
The patients also have been free of sickle cell disease’s associated pain attacks for a period of 11 months and seven months following therapy.
"Early results are showing very robust and very clinical pain relief, I'm excited to say that none of the patients who underwent this had any pain crisis. These initial results provide hope that this new technology will continue to show progress as we work toward creating a possible functional cure for this devastating and life-threatening disease," said Rabi Hanna, M.D., director of the pediatric blood and bone marrow transplant program at Cleveland Clinic Children’s and principal investigator at Cleveland Clinic Children's.
For most people with the disease, medications can modify disease severity and treat symptoms. However, despite current therapies, the average life of a sickle cell patient, is in the mid 40s.
A blood or marrow transplant can cure sickle cell disease, but the transplant often requires a sibling donor and has the potential for severe graft-versus-host disease, which is when donor bone marrow or stem cells attack the recipient.
The RUBY Trial aims to enroll 40 adult patients, ages 18 to 50, with severe sickle cell disease. Patients will be monitored closely after treatment for up to two years.